Milan Mrksich
Department of Biomedical Engineering and Chemistry, Northwestern University This email address is being protected from spambots. You need JavaScript enabled to view it.
This talk will describe an approach for synthesizing molecules that have sizes greater than 100 nm and yet are structurally perfectly defined. The approach relies on the selective and covalent reaction of an enzyme domain with an irreversible linker. Fusion proteins containing two or more of the enzyme domains are treated with linkers terminated in two or more of the irreversible inhibitors, leading to the rapid reaction of the partners and efficient assembly of the megamolecule. Several enzyme-inhibitor pairs have been developed, and used to prepare megamolecules that are linear, cyclic, branched, and that have molecular weights greater than 500,000 Dalton, and sizes greater than 100 nm (Figure 1). The talk will describe the use of this approach to create synthetic antibodies for therapeutic applications, and outline routes to a broad array of functional molecules.
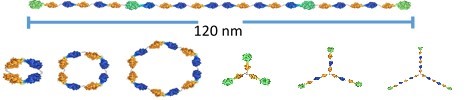
Figure 1. Megamolecules are prepared by reacting fusion proteins with molecular linkers that assemble by way of reactions of irreversible inhibitors with specific enzyme domains. Examples of megamolecules that can be assembled include linear structures (top), cyclic structures (left) and branched structures (right).
The megamolecules offer new approaches for preparing scaffolds that can spatially organize protein domains. This talk will describe the assembly of synthetic antibodies that position two (or more) Fab domains with control over the distance and orientation of the domains, as well as bispecific antibodies that present two distinct Fab domains. The talk will include a discussion of applications of this method to prepare nanoscale membranes having biological functions.